Structure Determination of Auxin Phytohormones.
 |
deutsche Version.
|
| © |
Copyright note. |
 |
Site-map. |
Structure Determination of Auxin Phytohormones. |
|
| T1 | T2 | E [kJ/mol] |
|---|---|---|
| 92.85° | 106.96° | 0.000 |
| 0.00° | 0.00° | 0.881 |
| 104.57° | 3.10° | 1.887 |
| 79.64° | -97.97° | 2.452 |
| 15.73° | 113.04° | 7.510 |
The potential energy surface of 4-methyl-indole-3-acetic acid (4-Me-IAA) contains five symmetry unique conformers (A, B, ..., E), the geometries of which are shown after this paragraph in the order of their energies. The values of T1 and T2 and the relative energies are collected in the table.
Similar to 4-Et-IAA, the global minimum A is not mirror-symmetrical but has a tilted acetic acid side chain. The carbonyl oxygen atom comes close enough to one of the methyl group hydrogen atoms (distance: 2.575 Å) to form a weak hydrogen bond (bond order: 0.018), which is weaker than the corresponding H-bond in the global minimum of 4-Et-IAA. The mirror symmetrical conformer B contains a different C-H···O=C hydrogen bond of similar strength (distance: 2.329 Å, bond order: 0.018), but also two repulsive H···H interactions between the methyl and the acetic acid side chain (distances: 2.405 Å). In the case of C, an interaction similar to the one in A is formed, the distance of which (2.767 Å), however, is too long for any H-bond character. In contrast, the interaction C-H···O of the methyl group with the hydroxylic oxygen atom in D has a very weak H-bond character (distance: 2.635 Å, bond order 0.011). Finally, E exhibits a similar C-H···O hydrogen bond between the OH group and the indole nucleus with a O···H distance of 2.563 Å and a bond order of 0.012.
The T1/T2-values of some conformers agree well with those of
unsubstituted IAA, others agree well
with those of 4-Cl-IAA. Also,
the reaction paths of 4-Me-IAA show characteristic elements of both,
IAA and 4-Cl-IAA.
In a qualitative sense, 4-Me-IAA thus is inbetween IAA and 4-Cl-IAA,
which has a fascinating equivalence in the biological activity
data: Rescher et al. studied the correlation between binding affinity and
maximum growth rate of meize coleoptil section
at the optimum concentration of 10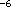 mol/l
for several compounds and determined the following order:
naphthalene-1-acetic acid > 4-Cl-IAA > 4-Me-IAA >
IAA > 4-Et-IAA > 2-Me-IAA.
In this order, 4-Me-IAA also takes a position between IAA and 4-Cl-IAA, although
this could be explained in part by the higher lipophilicity of the
chlorinated compounds.
mol/l
for several compounds and determined the following order:
naphthalene-1-acetic acid > 4-Cl-IAA > 4-Me-IAA >
IAA > 4-Et-IAA > 2-Me-IAA.
In this order, 4-Me-IAA also takes a position between IAA and 4-Cl-IAA, although
this could be explained in part by the higher lipophilicity of the
chlorinated compounds.