Structure Determination of Auxin Phytohormones.
 |
deutsche Version.
|
| © |
Copyright note. |
 |
Site-map. |
Structure Determination of Auxin Phytohormones. |
|
The potential energy surface of
4-chloro-indole-3-acetic acid
contains four symmetry unique local minima with a
cis-orientation of the COOH group, which are shown
on top of this paragraph in the order of their energies.
The 6-31G* optimized positions in
T1/T2-space
and the relative energies are collected in the table following this paragraph.
As in the case of unsubstituted indole-3-acetic acid (IAA),
the global minimum (A)
is mirror symmetrical and stabilized by a weak
C=O···H-C2 hydrogen bond.
Unlike IAA, the conformers with tilted side chain offer only
two different orientations for the COOH group, the torsion angles
of which differ by approximately 180°; these conformers are
second and third in energy.
The fourth symmetry unique local minimum (D)
is not only remarkable because
of its high relative energy. It is located at a T1/T2-position,
which is close to that of a saddle point in unsubstituted IAA.
Furthermore, D is no stable conformer:
the lowest potential barrier (in the reaction to A)
is only 0.452 kJ/mol, which is lower than the zero
point energy of the corresponding vibration mode (that
has an unscaled, harmonic frequency of 103.53 cm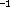 ).
).
| Conformer | T1 | T2 | E [kJ/mol] |
|---|---|---|---|
| A | 0.000° | 0.000° | 0.000 |
| B | 105.623° | -14.059° | 2.683 |
| C | 110.991° | 162.977° | 6.316 |
| D | 5.789° | 115.002° | 8.988 |
These four energy minima and their enantiomers are connected by a network of reaction paths, which contains the following connections (lower case symbolizing mirror images): A-B, A-b, A-C, A-c, A-D, A-d, B-c, B-D, C-D, and D-d. It should be noted that there is no direct reaction path between B and C, i.e., the COOH group cannot rotate freely in either of these conformers. Starting from B or C, an internal rotation of the COOH group is always coupled to a torsion of the acetic acid side chain.
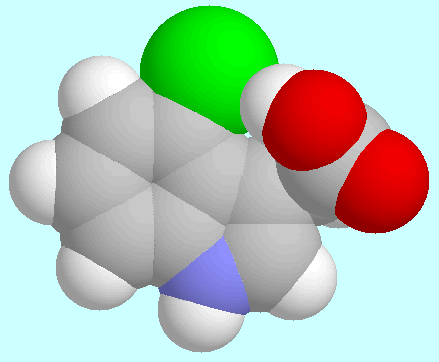
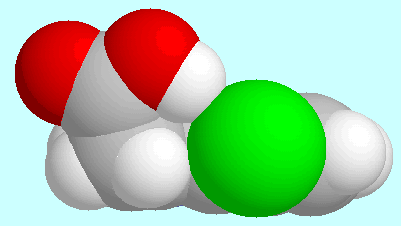 4-Cl-IAA has the possibility of forming a
O-H···Cl hydrogen bond that
closes a eight-membered ring. The geometry of this H-bonded conformer is
shown next to this paragraph, as seen from two different directions.
Due to the rigid nature of the indole nucleus,
the resulting hydrogen bond is considerably weaker than the
hydrogen bonds formed by
4-Cl-IAA has the possibility of forming a
O-H···Cl hydrogen bond that
closes a eight-membered ring. The geometry of this H-bonded conformer is
shown next to this paragraph, as seen from two different directions.
Due to the rigid nature of the indole nucleus,
the resulting hydrogen bond is considerably weaker than the
hydrogen bonds formed by
 -aminopentanoic acid or
-aminopentanoic acid or
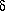 -hydroxypentanoic acid, which
also lead to eight-membered rings:
the 6-31G* relative energy of this conformer is 24.914 kJ/mol
and the hydrogen bond order is only 0.027.
-hydroxypentanoic acid, which
also lead to eight-membered rings:
the 6-31G* relative energy of this conformer is 24.914 kJ/mol
and the hydrogen bond order is only 0.027.