Intramolecular Hydrogen Bonds in Amino Alcohols.
 |
deutsche Version. |
| © |
Copyright note. |
 |
Site-map. |
Intramolecular Hydrogen Bonds in Amino Alcohols. |
|
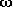 -amino-n-alkanoles
and
-amino-n-alkanoles
and 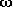 -amino
acids both form intramolecular
N···H-O hydrogen bonds, which are
stronger than any other intramolecular
interaction in these species. The direct comparison, which is given
below using one of the criteria for such a hydrogen bond, shows that this
bond is stronger in the
-amino
acids both form intramolecular
N···H-O hydrogen bonds, which are
stronger than any other intramolecular
interaction in these species. The direct comparison, which is given
below using one of the criteria for such a hydrogen bond, shows that this
bond is stronger in the  -amino acids.
It also shows that in both cases the various criteria for hydrogen bond
strength agree only up to the seven-membered ring formed
by the C
-amino acids.
It also shows that in both cases the various criteria for hydrogen bond
strength agree only up to the seven-membered ring formed
by the C -compound.
-compound.
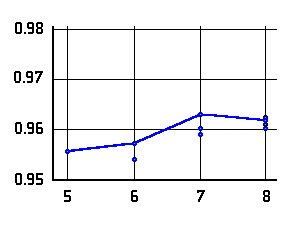
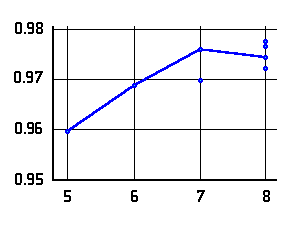
O-H distances (Å)
in N···H-O hydrogen bonded
 -amino
alcohols (left or top) and
-amino
alcohols (left or top) and  -amino
acids (right or bottom) as a function of the ring-size. The lines connect data
for conformers of lowest energy.
-amino
acids (right or bottom) as a function of the ring-size. The lines connect data
for conformers of lowest energy.
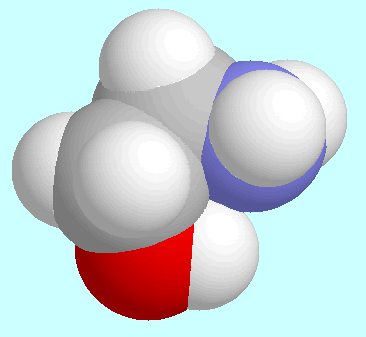
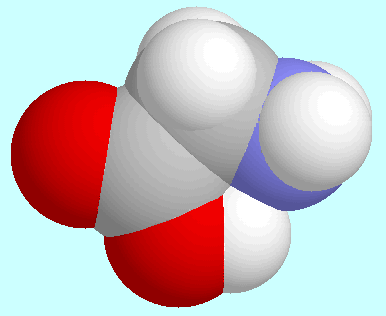 Another notable set of similarities and differences
between
Another notable set of similarities and differences
between  -amino
acids and
-amino
acids and  -amino
alcohols concerns the number and the geometry of
conformers with the N···H-O hydrogen bond.
Both 2-aminoethanol and glycine form only one symmetry-unique conformer,
which is asymmetrical in the former but essentially symmetrical in the latter.
The 2-aminoethanol potential energy surface contains two energetically
equivalent reaction paths that connect the N···H-O
bonded conformer with its enantiomer without breaking the hydrogen bond;
these reaction paths do not pass the mirror-symmetrical
conformation, in which the ring, that is closed by the hydrogen bond, becomes
planar as in glycine, but remain chiral from beginning to end.
In the case of the amino acids, this behaviour can be found in the
potential energy surface of the next homologue,
-amino
alcohols concerns the number and the geometry of
conformers with the N···H-O hydrogen bond.
Both 2-aminoethanol and glycine form only one symmetry-unique conformer,
which is asymmetrical in the former but essentially symmetrical in the latter.
The 2-aminoethanol potential energy surface contains two energetically
equivalent reaction paths that connect the N···H-O
bonded conformer with its enantiomer without breaking the hydrogen bond;
these reaction paths do not pass the mirror-symmetrical
conformation, in which the ring, that is closed by the hydrogen bond, becomes
planar as in glycine, but remain chiral from beginning to end.
In the case of the amino acids, this behaviour can be found in the
potential energy surface of the next homologue,
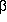 -alanine,
which forms a six-membered ring.
-alanine,
which forms a six-membered ring.
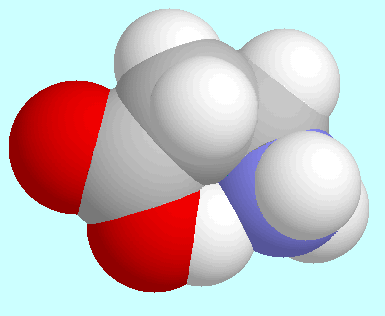 There is a significant difference between these two cases, though: in
There is a significant difference between these two cases, though: in
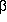 -alanine,
these reaction paths are the ones with the lowest energy
barrier for the hydrogen bonded conformer, whereas in 2-aminoethanol
several other reaction paths have a smaller barrier.
Analogously, 3-aminopropanol shows a similar picture as
-alanine,
these reaction paths are the ones with the lowest energy
barrier for the hydrogen bonded conformer, whereas in 2-aminoethanol
several other reaction paths have a smaller barrier.
Analogously, 3-aminopropanol shows a similar picture as 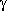 -aminobutyric
acid: two pairs of N···H-O bonded enantiomers
(A/A
-aminobutyric
acid: two pairs of N···H-O bonded enantiomers
(A/A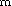 and B/B
and B/B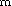 ), which are connected in a H-bond conserving
reaction cycle A-B-A
), which are connected in a H-bond conserving
reaction cycle A-B-A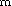 -B
-B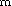 -A. Again, this cycle has
the lowest potential barriers in the amino acid but not in the
amino alcohol case.
-A. Again, this cycle has
the lowest potential barriers in the amino acid but not in the
amino alcohol case.
The comparison of 3-aminopropanol,
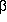 -alanine, and 3-aminopropanal
leads to the conclusion that the N···H-O hydrogen bonds,
although they
are the strongest of all hydrogen bonds formed in these systems, do
not have the biggest impact on the various potential surfaces.
Rather, the presence of the C=O group is the most influential
factor. The amino alcohol / amino acid comparison given above leads to
the same conclusion: the C=O group
forces an essentially planar arrangement in glycine,
which does not leave room for an enantiomer pair that is connected by
chiral reaction paths. This can only happen with one an additional
carbon atom, thus making the C
-alanine, and 3-aminopropanal
leads to the conclusion that the N···H-O hydrogen bonds,
although they
are the strongest of all hydrogen bonds formed in these systems, do
not have the biggest impact on the various potential surfaces.
Rather, the presence of the C=O group is the most influential
factor. The amino alcohol / amino acid comparison given above leads to
the same conclusion: the C=O group
forces an essentially planar arrangement in glycine,
which does not leave room for an enantiomer pair that is connected by
chiral reaction paths. This can only happen with one an additional
carbon atom, thus making the C amino alcohol equivalent to the C
amino alcohol equivalent to the C amino acid, and the C
amino acid, and the C amino alcohol equivalent to the C
amino alcohol equivalent to the C amino acid.
However, this equivalence of the number of H-bonded
conformers and reaction paths is limited by the different strength
of the N···H-O hydrogen bonds: the C
amino acid.
However, this equivalence of the number of H-bonded
conformers and reaction paths is limited by the different strength
of the N···H-O hydrogen bonds: the C amino alcohol, in which
N···H-O bond is strongest, is no longer an equivalent of
the C
amino alcohol, in which
N···H-O bond is strongest, is no longer an equivalent of
the C amino acid.
The different strength of the N···H-O hydrogen bonds
actually is a consequence of the
C=O group: it makes the hydrogen atom of the O-H group much more positive
than in an alcohol, and thus the H-bond stronger.
amino acid.
The different strength of the N···H-O hydrogen bonds
actually is a consequence of the
C=O group: it makes the hydrogen atom of the O-H group much more positive
than in an alcohol, and thus the H-bond stronger.
![]()
![]() 5-aminopentanol.
5-aminopentanol.
© publications
(our own).