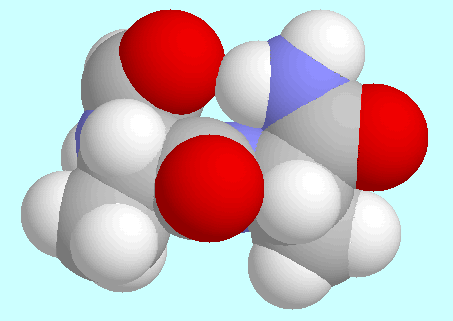
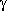 -turns
(7-membered rings closed by H-bond)
-turns
(7-membered rings closed by H-bond)|
M. Ramek, C.-H. Yu, and L. Schäfer,
Ab initio conformational analysis of the model tripeptide N-formyl-L-alanyl-L-alanine amide, Can. J. Chem. 76, 566-575 (1998). |
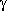 -turns
(7-membered rings closed by H-bond)
-turns
(7-membered rings closed by H-bond)
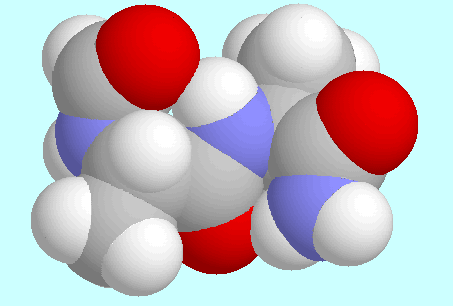 Extended Structure
second lowest in energy (Erel = 0.09 kJ/mol)
no hydrogen bonds, but two favourable electrostatic interactions
with parallel arrangement of the groups C=O and N-H (the so-called
C
Extended Structure
second lowest in energy (Erel = 0.09 kJ/mol)
no hydrogen bonds, but two favourable electrostatic interactions
with parallel arrangement of the groups C=O and N-H (the so-called
C -orientation)
-orientation)
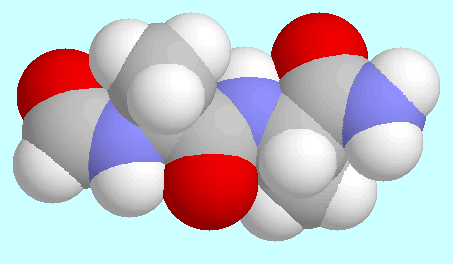 Half Extended Form
third in energy (Erel = 0.33 kJ/mol)
one
Half Extended Form
third in energy (Erel = 0.33 kJ/mol)
one 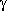 -turn,
one C
-turn,
one C -orientation
-orientation
 3
3
 -Loop
fourth in energy (Erel = 1.60 kJ/mol)
one
-Loop
fourth in energy (Erel = 1.60 kJ/mol)
one  -turn
(10-membered ring, closed by a O···H-N hydrogen bond)
repetition of this loop produces a
-turn
(10-membered ring, closed by a O···H-N hydrogen bond)
repetition of this loop produces a 
 -helix
-helix